Bereit, Ihr schönstes Ich zu entdecken? Finden Sie eine Cynosure Praxis in Ihrer Nähe.
Finden Sie eine PraxisNeueste Artikel

30
Nov
2021
Der neue Standard für RF-Microneedling mit verbesserter topischer Gewebedurchdringung Ein weiterer Meilenstein in der Zukunft der neuen Mikroneedling-Techniken wurde durch Potenza von Cynosure erreicht, und zwar als bisher erstes und einziges Microneedling-Gerät, welches mit dem Fusion Tip eine höhere Einbringung topischer Präparate in die Haut bietet. Potenza ist das weltweit erste RF-Microneedling-System, das monopolare und […]
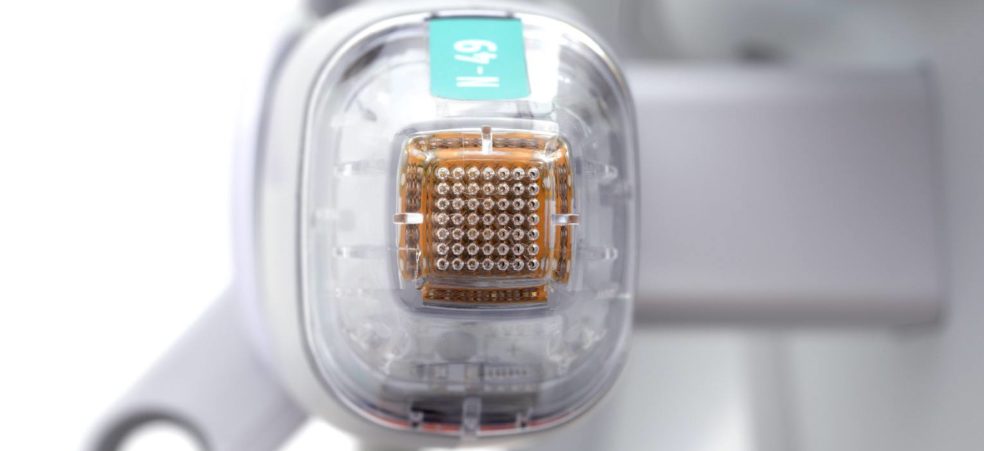
Bieten Sie Ihren Patienten die Ergebnisse, die sie verdienen. Potenza ist die neueste Innovation von Cynosure und definiert einen neuen Standard im RF-Microneedling. Die Hautrevitalisierung ist ein großer und schnell wachsender Markt. Der Weltmarkt für energiebasierte Plattformen für ästhetische Systeme erreichte im Jahr 2018 1,7 Milliarden US-Dollar, und diese Zahl wächst weiter. Microneedling gehört zu […]
Die NiSV regelt den Einsatz von so genannten Energy-Based-Devices (EDB). Hierzu zählen Radiofrequenzgeräte, die bei der Fettbekämpfung oder der Hautstraffung eingesetzt werden, sowie Magnet-Stimulationsgeräte zum Muskelaufbau. Die MDR regelt die Produktqualität von Geräten und Materialien, die in der Ästhetik eingesetzt werden und ist ab dem 26.05.2021 verbindlich anzuwenden. Die Verordnung stellt erhöhte Anforderungen an Schönheitsbehandlungen, […]